Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure
Related Articles: Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure
- 2 Introduction
- 3 Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure
- 3.1 The Atom: A Tiny World of Subatomic Particles
- 3.2 The Significance of Atomic Structure: Unlocking the Secrets of Chemistry
- 3.3 FAQs Regarding the Basic Makeup of an Atom
- 3.4 Tips for Understanding Atomic Structure
- 3.5 Conclusion
- 4 Closure
Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure
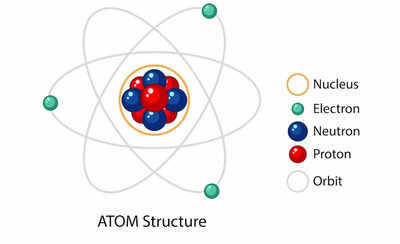
The world around us, from the tiniest speck of dust to the grandest stars, is composed of matter. And at the heart of all matter lies the atom, the fundamental building block of the universe. Understanding the structure of an atom is crucial for comprehending the behavior of matter, the properties of elements, and the intricate workings of chemistry. This article will delve into the basic makeup of an atom, exploring its components, their interactions, and the significance of this knowledge.
The Atom: A Tiny World of Subatomic Particles
Atoms are incredibly small, measuring only a few angstroms in diameter (one angstrom is one ten-billionth of a meter). Despite their diminutive size, atoms are complex entities, harboring a fascinating array of subatomic particles. These particles, bound together by powerful forces, define the atom’s unique properties and behavior.
1. The Nucleus: The Atom’s Core
At the center of every atom lies the nucleus, a dense and positively charged region containing two types of particles: protons and neutrons.
- Protons: Positively charged particles that contribute to the atom’s atomic number. The number of protons defines the element to which an atom belongs. For instance, all carbon atoms have six protons, while all oxygen atoms have eight.
- Neutrons: Neutrally charged particles that contribute to the atom’s atomic mass. While the number of protons determines the element, the number of neutrons can vary, leading to different isotopes of the same element.
The nucleus is held together by the strong nuclear force, a powerful force that overcomes the electrostatic repulsion between protons. This force is essential for the stability of the atom.
2. The Electron Cloud: A Realm of Negativity
Surrounding the nucleus is a cloud of negatively charged particles called electrons. These electrons are much smaller than protons and neutrons and occupy a region called the electron cloud.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. The arrangement of electrons in these shells determines the atom’s chemical properties and its ability to form bonds with other atoms.
The electron cloud is not a solid sphere but rather a probability distribution representing the likelihood of finding an electron at a particular point in space. The electrons are constantly in motion, creating a dynamic and complex structure around the nucleus.
The Significance of Atomic Structure: Unlocking the Secrets of Chemistry
Understanding the basic makeup of an atom is paramount for comprehending the fundamental principles of chemistry. Here’s how:
1. Chemical Reactions and Bonding: The arrangement of electrons in an atom’s outer shell, known as the valence shell, determines its reactivity. Atoms tend to achieve a stable configuration by gaining, losing, or sharing electrons with other atoms. This process forms chemical bonds, the foundation of all molecules and compounds.
2. Properties of Elements: The number of protons in an atom’s nucleus, its atomic number, defines the element to which it belongs. Each element possesses unique chemical and physical properties that are dictated by the arrangement of its electrons and the interactions between its nucleus and electron cloud.
3. Understanding the Periodic Table: The periodic table is a systematic arrangement of elements based on their atomic numbers and recurring chemical properties. The arrangement reflects the periodic trends in electron configuration and atomic size, providing a valuable tool for predicting and explaining the behavior of elements.
4. Applications in Science and Technology: The knowledge of atomic structure has revolutionized various fields, including:
- Materials Science: Understanding the arrangement of atoms in materials allows scientists to design and engineer materials with specific properties, leading to advancements in electronics, medicine, and energy production.
- Medicine: Medical imaging techniques like MRI and PET scans rely on the interaction of atoms with electromagnetic radiation, providing insights into the human body’s internal workings.
- Nuclear Physics: The study of the nucleus and its interactions with other particles has led to the development of nuclear power, nuclear medicine, and other applications.
FAQs Regarding the Basic Makeup of an Atom
Q: What is the difference between an atom and a molecule?
A: An atom is the smallest unit of an element that retains the chemical properties of that element. A molecule is formed when two or more atoms bond together. For example, a water molecule (H₂O) is composed of two hydrogen atoms and one oxygen atom.
Q: How are isotopes of an element different?
A: Isotopes of an element have the same number of protons (atomic number) but differ in the number of neutrons. This difference in neutron count affects the atom’s mass but not its chemical properties. For example, carbon-12 and carbon-14 are isotopes of carbon, with 6 and 8 neutrons, respectively.
Q: What are the different energy levels of electrons?
A: Electrons occupy specific energy levels or shells around the nucleus. These levels are quantized, meaning electrons can only exist at specific energy levels. The higher the energy level, the further the electron is from the nucleus.
Q: How do atoms form bonds?
A: Atoms form bonds by sharing, gaining, or losing electrons in their valence shell. This process leads to a more stable configuration for both atoms involved. There are three main types of chemical bonds: covalent, ionic, and metallic.
Tips for Understanding Atomic Structure
- Visualize the atom: Use models, diagrams, and animations to visualize the structure of an atom and the arrangement of its components.
- Focus on the key concepts: Understand the definitions of protons, neutrons, electrons, atomic number, and atomic mass.
- Practice with examples: Work through examples of different atoms and their electron configurations to solidify your understanding.
- Explore the periodic table: Use the periodic table to understand the trends in atomic properties and the relationships between elements.
Conclusion
The basic makeup of an atom, with its nucleus and electron cloud, is a fundamental concept in chemistry and physics. Understanding this structure unlocks the secrets of chemical reactions, the properties of elements, and the intricate workings of the universe. By delving into the world of atoms, we gain a deeper appreciation for the complexity and beauty of the matter that surrounds us.
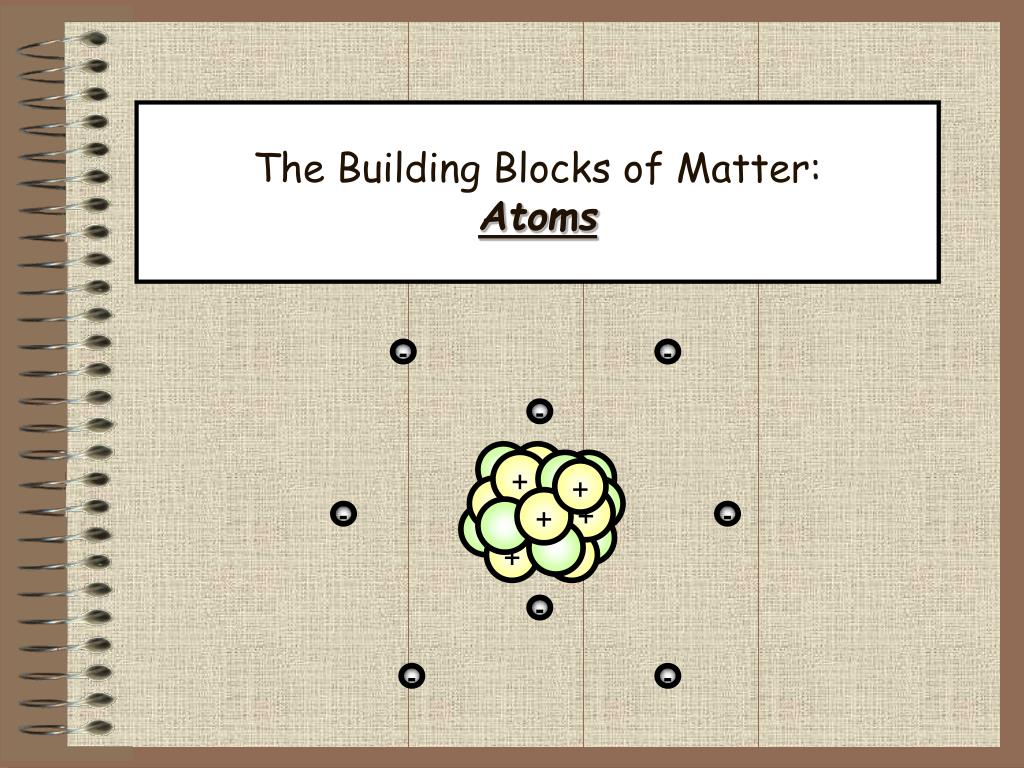






Closure
Thus, we hope this article has provided valuable insights into Delving into the Fundamental Building Blocks of Matter: A Comprehensive Guide to Atomic Structure. We appreciate your attention to our article. See you in our next article!